教学开放日,研究性小组在老师指导下探究CO2的性质,以下是有关实验装置.
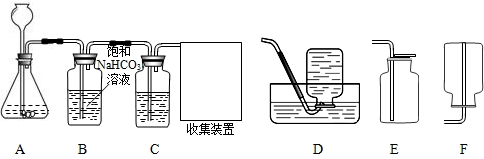
(1)实验室制取CO2,装置A中应盛装的药品是______.
A.碳酸钙和稀硫酸 B.碳酸钙和稀盐酸 C.锌粒和稀盐酸
(2)为收集纯净、干燥的CO2,将制得的气体通过B、C两个洗气瓶.B瓶用来除去CO2中的氯化氢,C瓶所盛的液体是______,收集装置应选用(填序号)______.
(3 )活动中老师演示了一个实验:将燃着的金属钠迅速伸入盛有干燥CO2的集气瓶里,钠在瓶中继续燃烧.反应后冷却,瓶内得到黑色颗粒和白色物质.
【提出问题】黑色颗粒和白色物质分别是什么呢?
【查阅资料】Na2O是白色固体,溶于水生成NaOH;CaCl2溶液呈中性.
【讨论猜想】大家认为黑色颗粒是______.对白色物质的成分,同学们提出四种猜想:
猜想l:可能是Na2O 猜想2:可能是Na2CO3
猜想3:可能是Na2 O和Na2CO3的混合物 猜想4:可能是NaOH
小敏认为猜想______不可能成立,理由是______.
【实验探究】取适量该白色物质溶于水制成溶液,分装于A、B两支试管中,进行如下实验:
①向A试管中加入澄清石灰水,产生白色沉淀,说明白色物质中有______.
②向B试管中加入过量CaCl2溶液,过滤;向滤液中滴加无色酚酞试液,无明显现象,说明白色物质中没有______.
【实验结论】通过探究,猜想(填序号)______成立.【能力提升】写出钠在CO2中燃烧的化学方程式______.




