(14分) 在一定体积的密闭容器中,进行如下化学反应:CO2(g)+H2(g)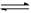 CO(g)+H2O(g),其化学平衡常数K与温度t的关系如下:
CO(g)+H2O(g),其化学平衡常数K与温度t的关系如下:
| t℃ | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
请回答下列问题:(1)该反应的化学平衡常数K =

。
(2)该反应为 反应。(填“吸热”或“放热”)
(3)800℃,固定容积的密闭容器中,放入混合物,起始浓度为c(CO)="0.01" mol/L, c(H2O)="0.03" mol/L, c(CO2)="0.01" mol/L, c(H2)="0.05" mol/L ,则反应开始时,H2O的消耗速率比生成速率 (填“大”、“小”或“不能确定”)
(4)830℃,在1 L的固定容器的密闭容器中放入2 mol CO2和1 mol H2,平衡后CO2的转化率为 , H2的转化率为 。